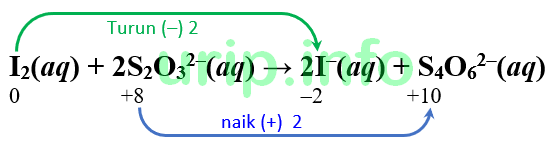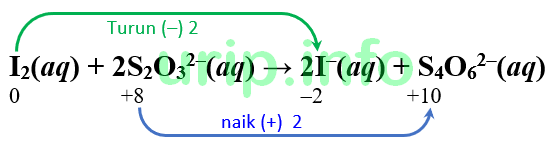I2 s2o32 i s4o62
https://littleroom.es/
музикэ молдовеняскэ де петречере мп3
I2 + S2O3{2-} = I{-} + S4O6{2-} - Balanced Chemical Equation. Balance I2 + S2O3 {2-} = I {-} + S4O6 {2-} Using the Algebraic Method. To balance the equation I2 + S2O3 {2-} = I {-} + S4O6 {2-} using the algebraic method step-by-step, you …. S2O32−(aq)+I2(aq)→S4O62−(aq)+I−(aq) - Socratic. 1 Answer anor277 Dec 2, 2017 Well, let us see.we write the individual redox reactions . Explanation: Reduction. 1 2 I 2 + e− → I − (i) Oxidation. 2S2O2− 3 → S4O2− 6 + 2e− …. I2 + S2O3{2-} = I{-} + S4O6 Redox Reaction - ChemicalAid. What is the oxidizing agent? The oxidizing agent, or oxidant/oxidizer, is a species that oxidizes other substances and gains electrons (i.e. oxidation number goes down). I2 …
urlop na pół etatu
水晶蝦
. I2 + S2O32 = I + S4O62 - Balanced Chemical Equation. Balance I2 + S2O32 = I + S4O62 Using the Algebraic Method
pastrav cu legume la cuptor
紫米露
. To balance the equation I2 + S2O32 = I + S4O62 using the algebraic method step-by-step, you must have experience …. S2O3 {2-} + I2 = I {-} + S4O6 {2-} - Balanced chemical equation .. Lets balance this equation using the algebraic method. First, we set all coefficients to variables a, b, c, d, 
a S 2 O 3 {2-} + b I 2 = c I {-} + d S 4 O 6 {2-} Now we write down …. Identify the oxidizing agent: #2"S"_2"O"_3 ^(2-) + "I"_2. Consequently, you can say that iodine, I2, is acting as an oxidizng agent because it is oxidizing the thiosulfate anion to the tetrathionate anion, S4O2− 6. S2O2− 3 …. Solved Write balanced net ionic equations for the …. See Answer Question: Write balanced net ionic equations for the following reactions in basic solution. A. S2O32− (aq)+I2 (aq)→S4O62− (aq)+I− (aq) Express your answer as a …. Balancing redox reactions by oxidation number change method. In the oxidation number change method the underlying principle is that the gain in the oxidation number (number of electrons) in one reactant must be equal to the loss in the …. Solved Consider the following reaction: I2 (aq) + 2 …. Chemistry Chemistry questions and answers Consider the following reaction: I2 (aq) + 2 S2O32− (aq) → S4O62− (aq) + 2 I− (aq) Look up or calculate the oxidation state of …. Answered: Is S2O32- + I2 → S4O62- + I- a… | bartleby
pt tirta alam segar produksi apa
アスキーアート 一覧 スマホ
. The iron content of hemoglobin is determined by destroying the hemoglobin molecule and producing small water-soluble ions and molecules. The iron in the aqueous solution is reduced to iron (II) ion and then titrated against potassium permanganate. In the titration, iron (ll) is oxidized to iron (III) and permanganate is reduced to manganese (II .. Solved The reaction of I2 with S2O32– is described by …. See Answer Question: The reaction of I2 with S2O32– is described by this equation: I2 + 2 S2O32– → 2I – + 2 S4O62– By using two stoichiometric mole ratios from this balanced …. Balancing redox reactions by the ion-electron method. Step 1. Write down the unbalanced equation (skeleton equation) of the chemical reaction. All reactants and products must be known. For a better result write the reaction in ionic …. Consider the redox reaction: I2(s) + S2O32- (aq) <---> 2I- (aq). Answer and Explanation: 1 Become a Study.com member to unlock this answer! Create your account View this answer A. answer: iodine I 2 + 2 e − → 2 I − B. answer: thiosulfate ion …. S2O3{2-} + I2 = S4O6{2-} + I{-} - Balanced Chemical Equation. Word Equation
高齢 者 熱 が 下がら ない
süd kimi ag qar ile yukle
Thiosulfate Ion + Diiodine = Tetrathionate (2-) + Iodide Ion. Two moles of Thiosulfate Ion [S 2 O 32-] and one mole of Diiodine [I 2] react to form one mole of …. S2O3{2-} + I3{-} = S4O6{2-} + I{-} - Balanced chemical equation .. Check the balance. Now, both sides have 4 H atoms and 2 O atoms. The equation is balanced. Balancing with algebraic method. This method uses algebraic equations to find the correct coefficients.
pokrowiec na wózek
cfare te bejme kur na iken zeri
. Manakah Unsur yang Mengalami Reaksi Oksidasi …. Rabu, 04 September 2019. Untuk menjawab pertanyaan manakah unsur yang mengalami reaksi oksidasi pada reaksi di bawah ini tentu harus tahu persis bilangan oksidasi (biloks) dan perubahan bilangan oksidasi …. Solved Identify the reducing agent in the following | Chegg.com
ο μεγάλοσ περίπατοσ του πέτρου εργασία
クレソ韓国語
. Identify the reducing agent in the following reaction: 2 S2O32–(aq) + I2(g) → S4O62–(aq) + 2 I–(aq) (SHOW WORK) S2O32–(aq) and I–(aq) I2(aq) I–(aq) S4O62–(aq) S2O32–(aq) There is no reducing agent. I–(aq) and I2(aq) S2O32–(aq) and …. S2O3{2-} + I2 = S4O6{2-} + I{-} - Balanced Chemical Equation. Step 4: Substitute Coefficients and Verify Result
Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Since there is an equal number of each element in the reactants and products of 2S2O3 {2-} + I2 = S4O6 {2-} + 2I {-}, the equation is balanced.. Solved 1. The thiosulfate ion (S2O32-) is oxidized by iodine - Chegg. Question: 1. The thiosulfate ion (S2O32-) is oxidized by iodine as follows: 2S2O32-(aq) + I2(aq) → S4O62-(aq) + 2I-(aq) In a certain experiment, 7.23×10-3 mol/L of S2O32- is consumed in the first 11.0 seconds of the reaction. Calculate the rate of consumption. Half equation for S2O32-/S4O62- couple - Physics Forums. The half equation for the S2O32-/S4O62- couple is S2O32- + 2H2O → 2SO42- + 4H+ + 2e-. 2. What is the purpose of writing half equations for redox reactions? Writing half equations allows us to track the transfer of electrons during a redox reaction and determine the oxidation states of each element involved
harga pasang kanopi per meter
mufulira college of nursing and midwifery
. 3.. Solved Consider the following reaction: I2 (aq) + 2 S2O32− - Chegg. Consider the following reaction: I2 (aq) + 2 S2O32− (aq) → S4O62− (aq) + 2 I− (aq) Look up or calculate the oxidation state of iodine in I2. There are 3 steps to solve this one. Who are the experts? Experts have been vetted by Chegg as …. I2 + S2O3 = I + S4O62 - Balanced Chemical Equation. Warning: 2 of the compounds in I2 + S2O3 = I + S4O62 are unrecognized. Verify the equation was entered correctly. ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. Reaction Information Disclaimer. I 2 +S 2 O 3 =I+S 4 O 62. Reactants. Diiodine - I 2.. S2o32−(aq)+i2(aq)→s4o62−(aq)+i−(aq) express your answer as …. The net ionic equation for the reaction between S2O32-(aq) and I2(aq) in basic solution is:. S2O32-(aq) + I2(aq) → S4O62-(aq) + I-(aq) In the given reaction, S2O32-(aq) reacts with I2(aq) in basic solution to form S4O62-(aq) and I-(aq). To write the net ionic equation, we need to consider only the species that participate in the chemical change.In …. Answered: Is S2O32- + I2 → S4O62- + I- a… | bartleby
pokoje gościnne na wydmie
gjakderdhje nga hunda dhe dhimbje koke
. The iron content of hemoglobin is determined by destroying the hemoglobin molecule and producing small water-soluble ions and molecules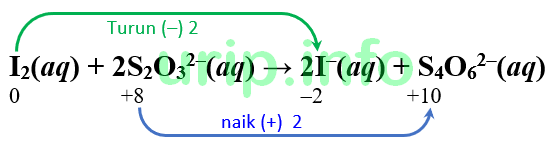
The iron in the aqueous solution is reduced to iron (II) ion and then titrated against potassium permanganate
In the titration, iron (ll) is oxidized to iron (III) and permanganate is reduced to manganese (II .. Balancing redox reactions by oxidation number change method. In the oxidation number change method the underlying principle is that the gain in the oxidation number (number of electrons) in one reactant must be equal to the loss in the oxidation number of the other reactant. Step 1. Write down the unbalanced equation (skeleton equation) of the chemical reaction. All reactants and products must be known.. In the reaction, I2+2S2O32−→2I−+S4O62−, equivalent mass. View Solution. Q 3
samira compagne de jonathan dassin
ფრანი
. In the reaction, I 2 +2S2O2− 3 → 2I − +S4O2− 6. equivalent weight of iodine is. View Solution. Q 4. Assertion : 1 mol of H 2SO4 is neutralised by 2 mol of N aOH; however, 1 equivalent of H 2SO4 is neutralised by 1 equivalent of N aOH. Reason: Equivalent mass of H 2SO4 is half of its molecular mass, however, the .. Solved Balance the following equation in acidic solution - Chegg. I2(s) + S2O32-(aq) → S4O62-(aq) + I-(aq) Balance the following equation in acidic solution using the lowest possible integers and give the coefficient of water. Here’s the best way to solve it.. Balance the following redox equation in acidic solution by using …. Redox Reactions: The key characteristic of a redox reaction is the transfer of electrons from one reactant to another. This results in the change in oxidation numbers wherein a positive change in the oxidation state indicates oxidation, while a negative change in the oxidation state indicates reduction.. Demi-équation électronique : S4O62-/S2O32- - YouTube. Vos questions en commentaire. Bon visionnage :)Cette vidéo montre la méthode pas à pas pour écrire la demi-équation électronique de réduction relative au cou.
sofás cómodos para la espalda
.